Abstract
Human malaria is a complex disease and a leading cause of mortality in children under 5 years of age. Plasmodium falciparum (Pf) is the agent responsible for cerebral malaria. Parasite infected erythrocytes are sequestered in the brain vasculature, disrupting the blood-brain-barrier, and with systemic inflammation leading to progressive brain edema. The precise pathophysiologic mechanism(s) underlying brain swelling in CM is not known. Recent work from our laboratories indicates that there is a role for bradykinin (BK) in fluid transport in human brain microvascular endothelial cells (Front Med 6:75, 2019).
We examined the role of bradykinin (BK) in pediatric CM. Initial studies showed recombinant falcipain-2, a cysteine protease contained in the parasite digestive vacuole, was inhibited by high molecular weight kininogen (HK), with an IC 50=36 nM. Further, falcipain-2, but not the related protease falcipain 3, hydrolyzed the chromogenic substrate S2302 (Pro-Phe-Arg-pNA) at pH 7.4 with an 88 nM K m. These results suggest that falcipain-2 has plasma kallikrein-like activity. HK is both an inhibitor and substrate of falcipain-2. Molar excess HK to falcipain-2 (ratio 8:1 to 2:1) blocked the proteolytic activity of the cysteine protease at pH 7.4. Equal molar falcipain-2 to HK (1:1) resulted in kallikrein-like cleavage of HK with stable BK liberation over 1 h. Molar excess falcipain-2 to HK (1:2 and greater) led to progressive HK cleavage into smaller proteins and peptides. The falcipain-2 major cleavages observed by N-terminal sequencing were in Domain 3 of the heavy chain of HK, the cysteine protease inhibitory region (I 292ASFSQNCDIYPGKDF 303, D 320IPTNSPELEETLT 334, and E 412KKIYPTVNCQPLG 425). P. falciparum trophozoite lysates completely hydrolyzed purified and plasma HK into a ~64 kDa heavy chain and ~46 kDa light chain in buffer containing EDTA, pepstatin, and PMSF. The cysteine proteinase inhibitor E64 blocked this cleavage, suggesting that the relevant activity was that of a cysteine protease.
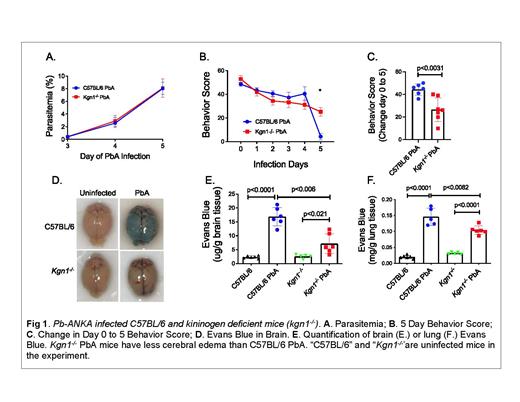
Plasma from Kenyan children presenting with CM (fever, parasitemia, coma) had evidence of circulating cHK, indicative of BK released from HK. Forty percent (8 of 20) of CM patients had no intact 120 kDa HK at hospital entry. In contrast, only 16% (3 of 8) of children with uncomplicated malaria had detectable cHK. In CM patients, the HK level before antimalarial treatment (58 ± 3.9 µg/ml) was significantly lower than the value after clinical recovery (69 ± 3.6 µg/ml; p<0.04) as measured by competitive ELISA. We also examined the roles of BK and HK in experimental cerebral malaria. 10 6 infected red blood cells with P. berghei ANKA were injected intraperitoneally into wild-type (C57BL/6) and total kininogen deficient (kgn1 -/-) C57BL/6 mice. The level of parasitemia on day 5 post-infection was ≥ 8% for both groups of mice (Figure 1). The kgn1 -/- mice had protected neuronal function measured by SHIRPA score relative to wild-type mice. Cerebral edema detected in wild- type mice by Evans Blue dye extravasation test was nearly completely attenuated in kgn1 -/- mice. Corroborative studies were performed in BK B2 receptor deleted (bdkrb2 -/-) mice. In mice with 15% parasitemia for both genotypes, there was significantly less neurologic function deterioration and a 30% reduction in cerebral Evans blue extravasation into brain parenchyma in the bdkrb2 -/- mice.
These data strongly suggest that falcipain-2 liberates BK from HK by acting like plasma kallikrein and in high concentrations destroys HK's cysteine protease inhibitory region. Some children with CM have in vivo evidence of prior HK proteolysis. Total kininogen deficiency protects mice from lethal experimental CM. Taken together, these data suggest that bradykinin is a proximal mediator of cerebral malaria.
McCrae: Dova, Novartis, Rigel, and Sanofi Genzyme: Consultancy; Sanofi, Novartis, Alexion, and Johnson & Johnson: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal